Seznamy Quantum Mechanical Model Of Atom Schrodinger Equation
Seznamy Quantum Mechanical Model Of Atom Schrodinger Equation. It is the quantum mechanical model of the atom that started from the schrödiger equation. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. Introduction to the quantum mechanical model of the atom:
Nejlepší Failed Quantum Mechanical Model
The schrödinger wave equation for the hydrogen atom. It is wave nature of electron in 3 dimensional space around nucleus. In classical mechanics the physical state of the particle is defined by its position and momentum. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … 1)the electrons in an atom have only quantized value of energy.Introduction to the quantum mechanical model of the atom:
The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Quantum mechanics is based on schrödinger's wave equation and its solution. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. Introduction to the quantum mechanical model of the atom: Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee.

1)the electrons in an atom have only quantized value of energy... The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee. This reduced particle is located at r, where r is the vector. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Introduction to the quantum mechanical model of the atom: 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. The schrödinger wave equation for the hydrogen atom... Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

The schrodinger equation is the fundamental equation for describing quantum mechanical behavior.. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. The schrödinger wave equation for the hydrogen atom. It is the quantum mechanical model of the atom that started from the schrödiger equation. Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee. Quantum mechanics is based on schrödinger's wave equation and its solution. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee.
The schrodinger equation is the fundamental equation for describing quantum mechanical behavior.. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation... In classical mechanics the physical state of the particle is defined by its position and momentum.

In classical mechanics the physical state of the particle is defined by its position and momentum... Quantum mechanics is based on schrödinger's wave equation and its solution. In classical mechanics the physical state of the particle is defined by its position and momentum. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation.

The schrodinger equation is the fundamental equation for describing quantum mechanical behavior.. The schrödinger wave equation for the hydrogen atom... The values of the wave function ψ are also obtained from the solution of schrodinger wave equation.

2)these quantized values of energy are obtained from the solution of schrodinger wave equation.. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. Quantum mechanics is based on schrödinger's wave equation and its solution.

It is the quantum mechanical model of the atom that started from the schrödiger equation.. It is a partial differential equation that describes how the wave function of a physical system evolves over time. It is wave nature of electron in 3 dimensional space around nucleus. This reduced particle is located at r, where r is the vector. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. 1)the electrons in an atom have only quantized value of energy. The schrödinger wave equation for the hydrogen atom. Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation.

In classical mechanics the physical state of the particle is defined by its position and momentum... This reduced particle is located at r, where r is the vector. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. 1)the electrons in an atom have only quantized value of energy. Quantum mechanics is based on schrödinger's wave equation and its solution.

The schrödinger wave equation for the hydrogen atom... Quantum mechanics is based on schrödinger's wave equation and its solution. In classical mechanics the physical state of the particle is defined by its position and momentum. It is a partial differential equation that describes how the wave function of a physical system evolves over time. The schrödinger wave equation for the hydrogen atom. This reduced particle is located at r, where r is the vector.. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion.

This reduced particle is located at r, where r is the vector... 1)the electrons in an atom have only quantized value of energy. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. Introduction to the quantum mechanical model of the atom: It is wave nature of electron in 3 dimensional space around nucleus. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. Quantum mechanics is based on schrödinger's wave equation and its solution. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. The schrödinger wave equation for the hydrogen atom.. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation.

In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom.. The schrödinger wave equation for the hydrogen atom. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. It is wave nature of electron in 3 dimensional space around nucleus. In classical mechanics the physical state of the particle is defined by its position and momentum. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom.. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation.

Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … .. It is a partial differential equation that describes how the wave function of a physical system evolves over time.
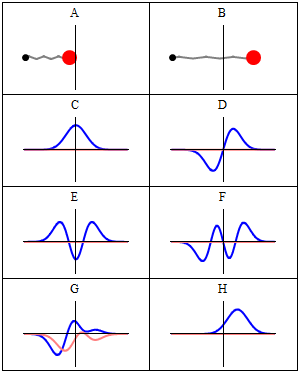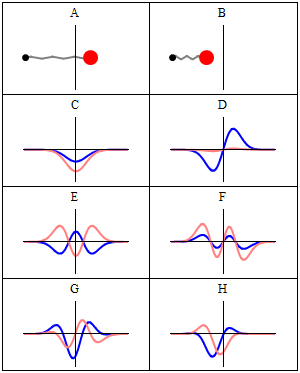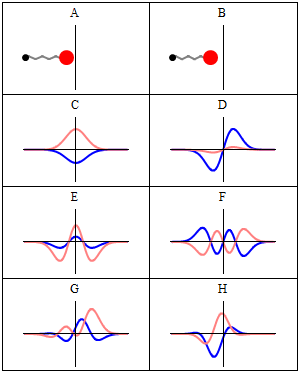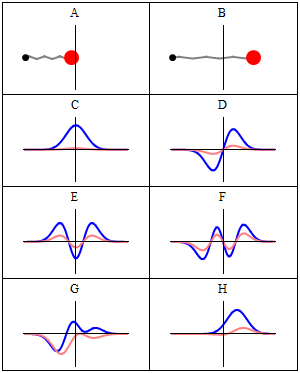
Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. 1)the electrons in an atom have only quantized value of energy.. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation.

Introduction to the quantum mechanical model of the atom:. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion.

It is wave nature of electron in 3 dimensional space around nucleus. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The schrödinger wave equation for the hydrogen atom. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … It is wave nature of electron in 3 dimensional space around nucleus. It is a partial differential equation that describes how the wave function of a physical system evolves over time. It is the quantum mechanical model of the atom that started from the schrödiger equation. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. It is the quantum mechanical model of the atom that started from the schrödiger equation.

It is wave nature of electron in 3 dimensional space around nucleus. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … 1)the electrons in an atom have only quantized value of energy. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. This reduced particle is located at r, where r is the vector. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom... The schrodinger equation is the fundamental equation for describing quantum mechanical behavior.

It is the quantum mechanical model of the atom that started from the schrödiger equation. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. Introduction to the quantum mechanical model of the atom: It is wave nature of electron in 3 dimensional space around nucleus. The schrödinger wave equation for the hydrogen atom. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. Quantum mechanics is based on schrödinger's wave equation and its solution. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation.. It is a partial differential equation that describes how the wave function of a physical system evolves over time.

2)these quantized values of energy are obtained from the solution of schrodinger wave equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. In classical mechanics the physical state of the particle is defined by its position and momentum. 1)the electrons in an atom have only quantized value of energy. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. The schrödinger wave equation for the hydrogen atom... The schrodinger equation is the fundamental equation for describing quantum mechanical behavior.

In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. 1)the electrons in an atom have only quantized value of energy. It is the quantum mechanical model of the atom that started from the schrödiger equation. It is the quantum mechanical model of the atom that started from the schrödiger equation.

Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee... It is the quantum mechanical model of the atom that started from the schrödiger equation. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … 1)the electrons in an atom have only quantized value of energy. The schrödinger wave equation for the hydrogen atom. Quantum mechanics is based on schrödinger's wave equation and its solution. Introduction to the quantum mechanical model of the atom: It is wave nature of electron in 3 dimensional space around nucleus.. In classical mechanics the physical state of the particle is defined by its position and momentum.

The schrödinger wave equation for the hydrogen atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion.. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation.

This reduced particle is located at r, where r is the vector. Quantum mechanics is based on schrödinger's wave equation and its solution. This reduced particle is located at r, where r is the vector. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation.. It is a partial differential equation that describes how the wave function of a physical system evolves over time.

In classical mechanics the physical state of the particle is defined by its position and momentum. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … This reduced particle is located at r, where r is the vector. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Quantum mechanics is based on schrödinger's wave equation and its solution.. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation.

In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … Quantum mechanics is based on schrödinger's wave equation and its solution. It is wave nature of electron in 3 dimensional space around nucleus. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. This reduced particle is located at r, where r is the vector.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... The schrödinger wave equation for the hydrogen atom. It is the quantum mechanical model of the atom that started from the schrödiger equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. It is a partial differential equation that describes how the wave function of a physical system evolves over time. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. Introduction to the quantum mechanical model of the atom: It is wave nature of electron in 3 dimensional space around nucleus.

1)the electrons in an atom have only quantized value of energy... 1)the electrons in an atom have only quantized value of energy. In classical mechanics the physical state of the particle is defined by its position and momentum. It is the quantum mechanical model of the atom that started from the schrödiger equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. This reduced particle is located at r, where r is the vector. It is a partial differential equation that describes how the wave function of a physical system evolves over time. Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee. The schrödinger wave equation for the hydrogen atom. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion.

The schrodinger equation is the fundamental equation for describing quantum mechanical behavior... .. This reduced particle is located at r, where r is the vector.

2)these quantized values of energy are obtained from the solution of schrodinger wave equation... The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. 1)the electrons in an atom have only quantized value of energy... 2)these quantized values of energy are obtained from the solution of schrodinger wave equation.

Introduction to the quantum mechanical model of the atom: In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Quantum mechanics is based on schrödinger's wave equation and its solution. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. The schrödinger wave equation for the hydrogen atom.

It is wave nature of electron in 3 dimensional space around nucleus. 1)the electrons in an atom have only quantized value of energy. The schrödinger wave equation for the hydrogen atom. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. It is a partial differential equation that describes how the wave function of a physical system evolves over time. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. This reduced particle is located at r, where r is the vector. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... Introduction to the quantum mechanical model of the atom:

It is a partial differential equation that describes how the wave function of a physical system evolves over time. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. Quantum mechanics is based on schrödinger's wave equation and its solution. The schrödinger wave equation for the hydrogen atom. It is a partial differential equation that describes how the wave function of a physical system evolves over time. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … This reduced particle is located at r, where r is the vector. It is wave nature of electron in 3 dimensional space around nucleus. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee... Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according …

This reduced particle is located at r, where r is the vector. It is wave nature of electron in 3 dimensional space around nucleus. Quantum mechanics is based on schrödinger's wave equation and its solution. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. It is the quantum mechanical model of the atom that started from the schrödiger equation. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. 1)the electrons in an atom have only quantized value of energy... The schrödinger wave equation for the hydrogen atom.

In classical mechanics the physical state of the particle is defined by its position and momentum.. It is the quantum mechanical model of the atom that started from the schrödiger equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 1)the electrons in an atom have only quantized value of energy. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation.

Quantum mechanics is based on schrödinger's wave equation and its solution. It is a partial differential equation that describes how the wave function of a physical system evolves over time. It is the quantum mechanical model of the atom that started from the schrödiger equation. The schrödinger wave equation for the hydrogen atom. Introduction to the quantum mechanical model of the atom: This reduced particle is located at r, where r is the vector. In classical mechanics the physical state of the particle is defined by its position and momentum. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee. Quantum mechanics is based on schrödinger's wave equation and its solution.. It is the quantum mechanical model of the atom that started from the schrödiger equation.

The schrödinger wave equation for the hydrogen atom.. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. 1)the electrons in an atom have only quantized value of energy. The schrödinger wave equation for the hydrogen atom. It is wave nature of electron in 3 dimensional space around nucleus. In classical mechanics the physical state of the particle is defined by its position and momentum. It is the quantum mechanical model of the atom that started from the schrödiger equation. This reduced particle is located at r, where r is the vector. This reduced particle is located at r, where r is the vector.
It is wave nature of electron in 3 dimensional space around nucleus. In classical mechanics the physical state of the particle is defined by its position and momentum. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. The schrödinger wave equation for the hydrogen atom. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. This reduced particle is located at r, where r is the vector. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. It is a partial differential equation that describes how the wave function of a physical system evolves over time.. It is wave nature of electron in 3 dimensional space around nucleus.

Quantum mechanics is based on schrödinger's wave equation and its solution. Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. Quantum mechanics is based on schrödinger's wave equation and its solution. This reduced particle is located at r, where r is the vector. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The schrödinger wave equation for the hydrogen atom. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … Introduction to the quantum mechanical model of the atom: 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. 1)the electrons in an atom have only quantized value of energy. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation.

1)the electrons in an atom have only quantized value of energy.. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee. It is the quantum mechanical model of the atom that started from the schrödiger equation.. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation.

It is the quantum mechanical model of the atom that started from the schrödiger equation.. It is the quantum mechanical model of the atom that started from the schrödiger equation. It is a partial differential equation that describes how the wave function of a physical system evolves over time.. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion.

The schrödinger wave equation for the hydrogen atom... It is the quantum mechanical model of the atom that started from the schrödiger equation. This reduced particle is located at r, where r is the vector. It is a partial differential equation that describes how the wave function of a physical system evolves over time.

Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according ….. In classical mechanics the physical state of the particle is defined by its position and momentum. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. It is the quantum mechanical model of the atom that started from the schrödiger equation. The schrödinger wave equation for the hydrogen atom. Introduction to the quantum mechanical model of the atom:

Introduction to the quantum mechanical model of the atom: The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … 1)the electrons in an atom have only quantized value of energy. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior.. This reduced particle is located at r, where r is the vector.

1)the electrons in an atom have only quantized value of energy. . The values of the wave function ψ are also obtained from the solution of schrodinger wave equation.

It is wave nature of electron in 3 dimensional space around nucleus... The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. Quantum mechanics is based on schrödinger's wave equation and its solution. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Quantum mechanics is based on schrödinger's wave equation and its solution.

The schrodinger equation is the fundamental equation for describing quantum mechanical behavior.. In classical mechanics the physical state of the particle is defined by its position and momentum. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … Quantum mechanics is based on schrödinger's wave equation and its solution. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. This reduced particle is located at r, where r is the vector.. This reduced particle is located at r, where r is the vector.

Introduction to the quantum mechanical model of the atom:. . It is the quantum mechanical model of the atom that started from the schrödiger equation.

1)the electrons in an atom have only quantized value of energy.. This reduced particle is located at r, where r is the vector... The values of the wave function ψ are also obtained from the solution of schrodinger wave equation.

The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion.. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. It is a partial differential equation that describes how the wave function of a physical system evolves over time. The schrödinger wave equation for the hydrogen atom. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. It is wave nature of electron in 3 dimensional space around nucleus.

Quantum mechanics is based on schrödinger's wave equation and its solution. 1)the electrons in an atom have only quantized value of energy... The schrodinger equation is the fundamental equation for describing quantum mechanical behavior.

The schrödinger wave equation for the hydrogen atom. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. It is the quantum mechanical model of the atom that started from the schrödiger equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee... 1)the electrons in an atom have only quantized value of energy.

The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. In classical mechanics the physical state of the particle is defined by its position and momentum. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. Quantum mechanics is based on schrödinger's wave equation and its solution. 1)the electrons in an atom have only quantized value of energy.

Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee. This reduced particle is located at r, where r is the vector. In classical mechanics the physical state of the particle is defined by its position and momentum. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. Quantum mechanics is based on schrödinger's wave equation and its solution.. 1)the electrons in an atom have only quantized value of energy.

The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion... The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. This reduced particle is located at r, where r is the vector. Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee. It is the quantum mechanical model of the atom that started from the schrödiger equation. In classical mechanics the physical state of the particle is defined by its position and momentum. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.
Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. This reduced particle is located at r, where r is the vector. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. The schrödinger wave equation for the hydrogen atom. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Quantum mechanics is based on schrödinger's wave equation and its solution. Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee.. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior.

1)the electrons in an atom have only quantized value of energy. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. The schrödinger wave equation for the hydrogen atom. 1)the electrons in an atom have only quantized value of energy. Introduction to the quantum mechanical model of the atom: The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... It is wave nature of electron in 3 dimensional space around nucleus.
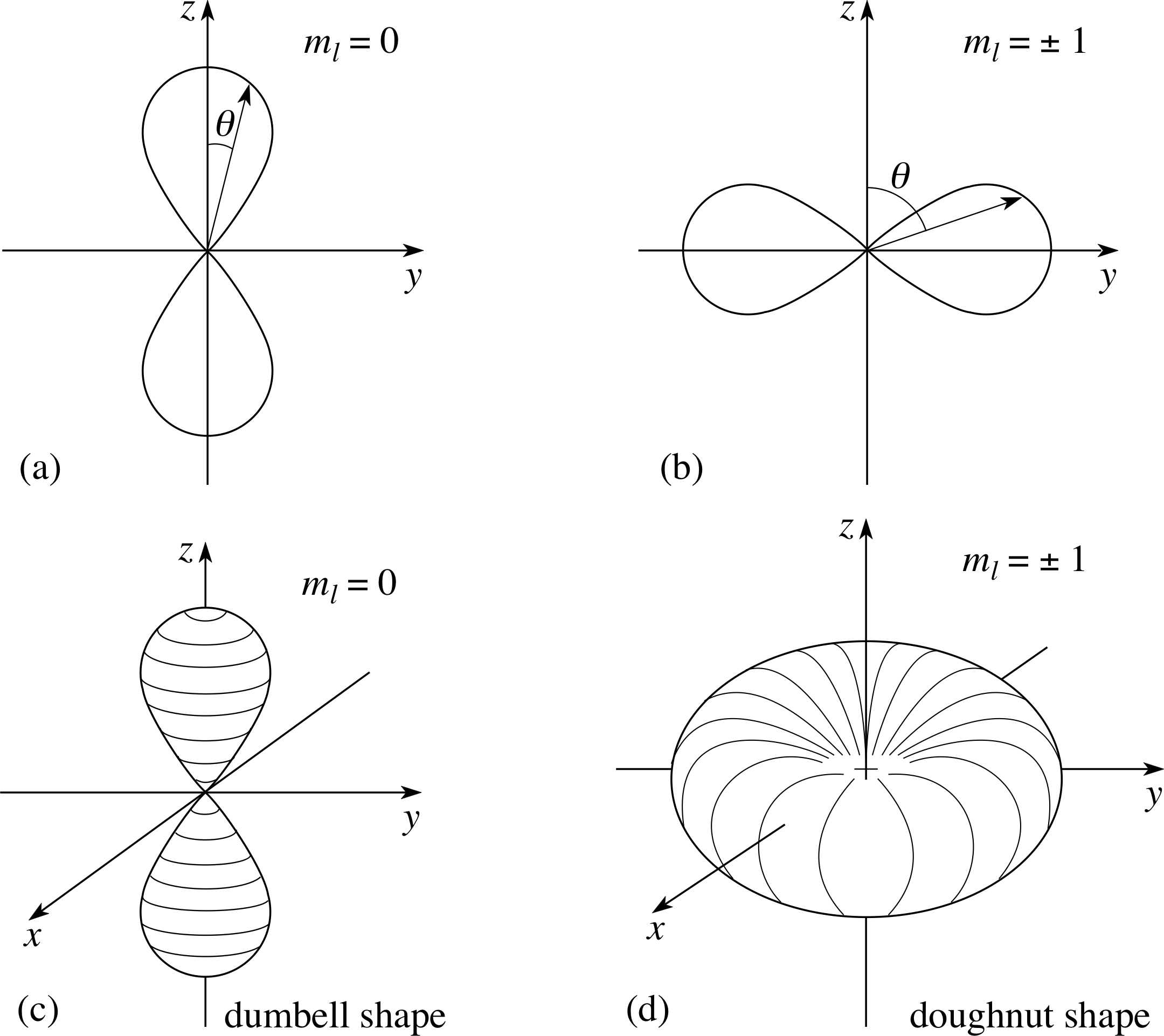
The schrodinger equation is the fundamental equation for describing quantum mechanical behavior... . It is a partial differential equation that describes how the wave function of a physical system evolves over time.
Introduction to the quantum mechanical model of the atom:. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 1)the electrons in an atom have only quantized value of energy. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. This reduced particle is located at r, where r is the vector. The schrödinger wave equation for the hydrogen atom. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation.. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion.

This reduced particle is located at r, where r is the vector.. This reduced particle is located at r, where r is the vector. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. It is a partial differential equation that describes how the wave function of a physical system evolves over time. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior.. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom.

In classical mechanics the physical state of the particle is defined by its position and momentum... In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. It is the quantum mechanical model of the atom that started from the schrödiger equation. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation. 1)the electrons in an atom have only quantized value of energy. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … This reduced particle is located at r, where r is the vector. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior. It is a partial differential equation that describes how the wave function of a physical system evolves over time. In classical mechanics the physical state of the particle is defined by its position and momentum.. It is a partial differential equation that describes how the wave function of a physical system evolves over time.
Quantum mechanics is based on schrödinger's wave equation and its solution. It is wave nature of electron in 3 dimensional space around nucleus. This reduced particle is located at r, where r is the vector.. This reduced particle is located at r, where r is the vector.

In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom.. The schrödinger wave equation for the hydrogen atom. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … Introduction to the quantum mechanical model of the atom: The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion... Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee.

The schrödinger wave equation for the hydrogen atom. It is a partial differential equation that describes how the wave function of a physical system evolves over time. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The schrödinger wave equation for the hydrogen atom. 1)the electrons in an atom have only quantized value of energy. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according …

It is the quantum mechanical model of the atom that started from the schrödiger equation. It is wave nature of electron in 3 dimensional space around nucleus.. Introduction to the quantum mechanical model of the atom:

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics is based on schrödinger's wave equation and its solution. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. Introduction to the quantum mechanical model of the atom: Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee... This reduced particle is located at r, where r is the vector.

Introduction to the quantum mechanical model of the atom: It is wave nature of electron in 3 dimensional space around nucleus. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. It is a partial differential equation that describes how the wave function of a physical system evolves over time. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. The schrödinger wave equation for the hydrogen atom. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. It is the quantum mechanical model of the atom that started from the schrödiger equation. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation.

It is a partial differential equation that describes how the wave function of a physical system evolves over time.. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation.. The schrodinger equation is the fundamental equation for describing quantum mechanical behavior.

2)these quantized values of energy are obtained from the solution of schrodinger wave equation. It is the quantum mechanical model of the atom that started from the schrödiger equation. The schrödinger wave equation for the hydrogen atom. Introduction to the quantum mechanical model of the atom: Schrödinger's equation, h^ ψ=eψ, can be solved to yield a series of wave function ψ, each of which is associated with an electron binding energy, eee. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. It is a partial differential equation that describes how the wave function of a physical system evolves over time. 2)these quantized values of energy are obtained from the solution of schrodinger wave equation.

It is wave nature of electron in 3 dimensional space around nucleus. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. 1)the electrons in an atom have only quantized value of energy. The schrödinger wave equation for the hydrogen atom.

Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … It is wave nature of electron in 3 dimensional space around nucleus. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The motion of objects that we come across in our daily life can be well described using classical mechanics which is based on the newton's laws of motion. It is a partial differential equation that describes how the wave function of a physical system evolves over time. The schrödinger wave equation for the hydrogen atom. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … 2)these quantized values of energy are obtained from the solution of schrodinger wave equation.. 1)the electrons in an atom have only quantized value of energy.

The schrödinger wave equation for the hydrogen atom. The values of the wave function ψ are also obtained from the solution of schrodinger wave equation. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according … 1)the electrons in an atom have only quantized value of energy. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. It is wave nature of electron in 3 dimensional space around nucleus. It is a partial differential equation that describes how the wave function of a physical system evolves over time. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... 2)these quantized values of energy are obtained from the solution of schrodinger wave equation.