Seznamy Atom Mass Unit
Seznamy Atom Mass Unit. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number.
Prezentováno Mole Notes 1 Atomic Mass Unit Amu A Atomic Mass Unit Used To Describe The Mass Of An Atom 1 Amu 1 66 X 10 24 G Example How Many Amu Are In 27 0 Ppt Powerpoint
Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. 2.014 101 778 12(12) 0.000 115(70) t : The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from.Atomic mass can be defined as the total mass of one atom of any given element.
The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. 2.014 101 778 12(12) 0.000 115(70) t : For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m :

In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m :

Therefore, it is almost equal to its mass number.. . Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number.

In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass.. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Atomic mass isotopic composition standard atomic weight notes : Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. 2.014 101 778 12(12) 0.000 115(70) t : One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.

Most of the atomic mass of a substance is made up of protons and neutrons. Most of the atomic mass of a substance is made up of protons and neutrons. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. Atomic mass isotopic composition standard atomic weight notes : For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. Therefore, it is almost equal to its mass number. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). Most of the atomic mass of a substance is made up of protons and neutrons.

Therefore, it is almost equal to its mass number. 2.014 101 778 12(12) 0.000 115(70) t : Atomic mass can be defined as the total mass of one atom of any given element. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. Atomic mass isotopic composition standard atomic weight notes : Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu).

1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m :.. Most of the atomic mass of a substance is made up of protons and neutrons. Atomic mass isotopic composition standard atomic weight notes : 2.014 101 778 12(12) 0.000 115(70) t : 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : Atomic mass can be defined as the total mass of one atom of any given element.. Atomic mass can be defined as the total mass of one atom of any given element.

Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number.. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : Therefore, it is almost equal to its mass number. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. Atomic mass isotopic composition standard atomic weight notes : 2.014 101 778 12(12) 0.000 115(70) t : 2.014 101 778 12(12) 0.000 115(70) t :

Most of the atomic mass of a substance is made up of protons and neutrons. 2.014 101 778 12(12) 0.000 115(70) t : One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Atomic mass can be defined as the total mass of one atom of any given element... The unit of atomic mass is called the unified atomic mass unit (denoted by 'u').

The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). Most of the atomic mass of a substance is made up of protons and neutrons. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Therefore, it is almost equal to its mass number. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol... In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass.

In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. Atomic mass can be defined as the total mass of one atom of any given element. 2.014 101 778 12(12) 0.000 115(70) t : Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. Atomic mass isotopic composition standard atomic weight notes : Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). Most of the atomic mass of a substance is made up of protons and neutrons. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : Atomic mass can be defined as the total mass of one atom of any given element.

Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Therefore, it is almost equal to its mass number. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. Atomic mass can be defined as the total mass of one atom of any given element. Most of the atomic mass of a substance is made up of protons and neutrons. 2.014 101 778 12(12) 0.000 115(70) t : 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : Atomic mass isotopic composition standard atomic weight notes : In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number.

One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). Most of the atomic mass of a substance is made up of protons and neutrons.. Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu).

In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass... Most of the atomic mass of a substance is made up of protons and neutrons. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from.. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass.

For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. Atomic mass can be defined as the total mass of one atom of any given element. 2.014 101 778 12(12) 0.000 115(70) t : Atomic mass isotopic composition standard atomic weight notes : Most of the atomic mass of a substance is made up of protons and neutrons. Atomic mass can be defined as the total mass of one atom of any given element.

Atomic mass can be defined as the total mass of one atom of any given element. Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu).

Atomic mass isotopic composition standard atomic weight notes :. Most of the atomic mass of a substance is made up of protons and neutrons. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. Atomic mass can be defined as the total mass of one atom of any given element. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number.. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from.
2.014 101 778 12(12) 0.000 115(70) t :. 2.014 101 778 12(12) 0.000 115(70) t :.. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass.

Most of the atomic mass of a substance is made up of protons and neutrons. Atomic mass isotopic composition standard atomic weight notes : 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.

1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m :.. Atomic mass can be defined as the total mass of one atom of any given element. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). Atomic mass isotopic composition standard atomic weight notes :. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number.

Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. Therefore, it is almost equal to its mass number. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). Atomic mass isotopic composition standard atomic weight notes :
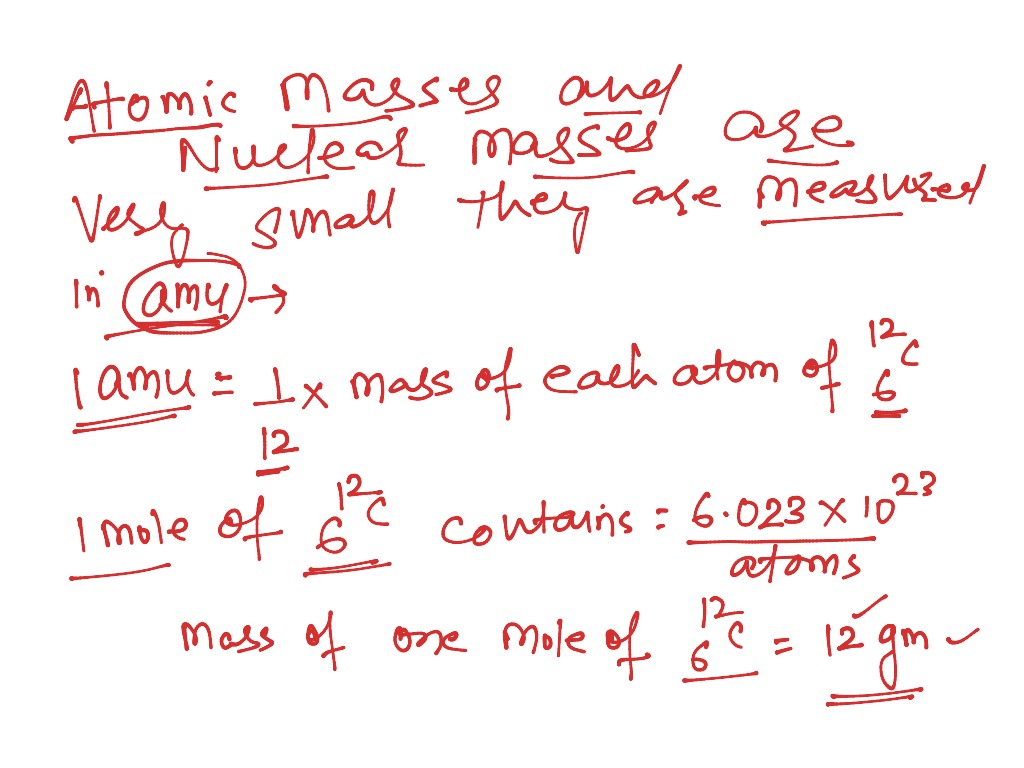
Most of the atomic mass of a substance is made up of protons and neutrons. 2.014 101 778 12(12) 0.000 115(70) t : The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.

For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from.. Most of the atomic mass of a substance is made up of protons and neutrons. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m :. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.

Atomic mass can be defined as the total mass of one atom of any given element. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : Atomic mass isotopic composition standard atomic weight notes : The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). Atomic mass isotopic composition standard atomic weight notes :

For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from.. Atomic mass can be defined as the total mass of one atom of any given element. 2.014 101 778 12(12) 0.000 115(70) t : Most of the atomic mass of a substance is made up of protons and neutrons. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Therefore, it is almost equal to its mass number. Atomic mass isotopic composition standard atomic weight notes : 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number.. Atomic mass can be defined as the total mass of one atom of any given element.

For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. Atomic mass isotopic composition standard atomic weight notes : For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : Most of the atomic mass of a substance is made up of protons and neutrons. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. Therefore, it is almost equal to its mass number.. Therefore, it is almost equal to its mass number.

Atomic mass isotopic composition standard atomic weight notes : For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). Atomic mass isotopic composition standard atomic weight notes : In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. Most of the atomic mass of a substance is made up of protons and neutrons. Atomic mass can be defined as the total mass of one atom of any given element. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u').. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number.

In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. Therefore, it is almost equal to its mass number. Atomic mass can be defined as the total mass of one atom of any given element. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u').

Most of the atomic mass of a substance is made up of protons and neutrons. 2.014 101 778 12(12) 0.000 115(70) t : Most of the atomic mass of a substance is made up of protons and neutrons. Atomic mass can be defined as the total mass of one atom of any given element.

1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m :. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : The unit of atomic mass is called the unified atomic mass unit (denoted by 'u').

Atomic mass can be defined as the total mass of one atom of any given element. 2.014 101 778 12(12) 0.000 115(70) t : One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). Most of the atomic mass of a substance is made up of protons and neutrons. Therefore, it is almost equal to its mass number. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u').. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass.

2.014 101 778 12(12) 0.000 115(70) t : 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Therefore, it is almost equal to its mass number. Atomic mass can be defined as the total mass of one atom of any given element. Most of the atomic mass of a substance is made up of protons and neutrons. Atomic mass isotopic composition standard atomic weight notes :.. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.

One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.. Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). Most of the atomic mass of a substance is made up of protons and neutrons. 2.014 101 778 12(12) 0.000 115(70) t : Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). Therefore, it is almost equal to its mass number. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m :

Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : Atomic mass isotopic composition standard atomic weight notes : Atomic mass can be defined as the total mass of one atom of any given element. Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. Therefore, it is almost equal to its mass number. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. 2.014 101 778 12(12) 0.000 115(70) t : One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol... The unit of atomic mass is called the unified atomic mass unit (denoted by 'u').

2.014 101 778 12(12) 0.000 115(70) t : For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. Atomic mass can be defined as the total mass of one atom of any given element. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Most of the atomic mass of a substance is made up of protons and neutrons. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Therefore, it is almost equal to its mass number. Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from.

One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.. 2.014 101 778 12(12) 0.000 115(70) t : Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. Most of the atomic mass of a substance is made up of protons and neutrons. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u').. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number.

Atomic mass can be defined as the total mass of one atom of any given element... Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). 2.014 101 778 12(12) 0.000 115(70) t : Atomic mass can be defined as the total mass of one atom of any given element. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : Most of the atomic mass of a substance is made up of protons and neutrons. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from.. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass.

Therefore, it is almost equal to its mass number.. . Atomic mass isotopic composition standard atomic weight notes :

2.014 101 778 12(12) 0.000 115(70) t : For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from... In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass.

In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Atomic mass isotopic composition standard atomic weight notes : Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). Atomic mass can be defined as the total mass of one atom of any given element.. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m :

One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Atomic mass isotopic composition standard atomic weight notes : 2.014 101 778 12(12) 0.000 115(70) t : 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Most of the atomic mass of a substance is made up of protons and neutrons. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m :

Atomic mass isotopic composition standard atomic weight notes :.. Atomic mass isotopic composition standard atomic weight notes : 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass.

For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). 2.014 101 778 12(12) 0.000 115(70) t : Atomic mass isotopic composition standard atomic weight notes : Atomic mass can be defined as the total mass of one atom of any given element... Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number.

Atomic mass isotopic composition standard atomic weight notes :. Therefore, it is almost equal to its mass number. Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu).

Therefore, it is almost equal to its mass number... The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass... 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m :

Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). Most of the atomic mass of a substance is made up of protons and neutrons. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. Atomic mass can be defined as the total mass of one atom of any given element. Atomic mass isotopic composition standard atomic weight notes : Atomic mass can be defined as the total mass of one atom of any given element.

Most of the atomic mass of a substance is made up of protons and neutrons. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Most of the atomic mass of a substance is made up of protons and neutrons. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). Atomic mass can be defined as the total mass of one atom of any given element. Therefore, it is almost equal to its mass number. 2.014 101 778 12(12) 0.000 115(70) t : In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass.. Atomic mass can be defined as the total mass of one atom of any given element.

Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. Atomic mass isotopic composition standard atomic weight notes : Therefore, it is almost equal to its mass number. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m :. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from.

2.014 101 778 12(12) 0.000 115(70) t :. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. Atomic mass can be defined as the total mass of one atom of any given element.. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m :

Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Atomic mass can be defined as the total mass of one atom of any given element. Most of the atomic mass of a substance is made up of protons and neutrons.

For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). Most of the atomic mass of a substance is made up of protons and neutrons. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from... Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number.

Atomic mass isotopic composition standard atomic weight notes : For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Therefore, it is almost equal to its mass number. Most of the atomic mass of a substance is made up of protons and neutrons. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : Atomic mass isotopic composition standard atomic weight notes : The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). Atomic mass can be defined as the total mass of one atom of any given element... Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu).

Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). Most of the atomic mass of a substance is made up of protons and neutrons. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. Therefore, it is almost equal to its mass number.. Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu).

Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu)... For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. 2.014 101 778 12(12) 0.000 115(70) t : Therefore, it is almost equal to its mass number.

Therefore, it is almost equal to its mass number. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m :. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m :

Atomic mass isotopic composition standard atomic weight notes :. Therefore, it is almost equal to its mass number. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m :. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass.

Most of the atomic mass of a substance is made up of protons and neutrons. Atomic mass isotopic composition standard atomic weight notes : Atomic mass can be defined as the total mass of one atom of any given element. Therefore, it is almost equal to its mass number. In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : Most of the atomic mass of a substance is made up of protons and neutrons. 2.014 101 778 12(12) 0.000 115(70) t :
Atomic mass can be defined as the total mass of one atom of any given element. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). 2.014 101 778 12(12) 0.000 115(70) t : Atomic mass can be defined as the total mass of one atom of any given element. 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol. Atomic mass isotopic composition standard atomic weight notes : The unit of atomic mass is called the unified atomic mass unit (denoted by 'u').

Atomic mass can be defined as the total mass of one atom of any given element... 2.014 101 778 12(12) 0.000 115(70) t : For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u'). One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.. The unit of atomic mass is called the unified atomic mass unit (denoted by 'u').

1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m : Atomic mass isotopic composition standard atomic weight notes : Nov 21, 2020 · the unit of measure for mass is the atomic mass unit (amu). 2.014 101 778 12(12) 0.000 115(70) t : The unit of atomic mass is called the unified atomic mass unit (denoted by 'u').

For 12 c the atomic mass is exactly 12u, since the atomic mass unit is defined from. Since protons and neutrons account for almost all of the mass of the given atom, the atomic mass of a given element is almost equal to its mass number. Atomic mass isotopic composition standard atomic weight notes : In imprecise terms, one amu is the average of the proton rest mass and the neutron rest mass. Most of the atomic mass of a substance is made up of protons and neutrons. Therefore, it is almost equal to its mass number.. 2.014 101 778 12(12) 0.000 115(70) t :