Kolekce Neutral Nitrogen Atom
Kolekce Neutral Nitrogen Atom. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he.
Nejlepší 6 Atomic Partial Charges For The Neutral And Charged Form Of Download Scientific Diagram
This element is found in group 15 and period 2 of the periodic table of the elements. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is …24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7.
With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is … Atomic number = number of electrons = number of protons = 7. 08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius). Hence, the given element has 7 protons, 7 electrons and 7 neutrons. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas.

29.09.2019 · this is also related to protons and neutrons present in an atom... Atomic number = number of electrons = number of protons = 7. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. There are 118 elements in the periodic table. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge.. Hence, the given element has 7 protons, 7 electrons and 7 neutrons.

Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge... .. There are 118 elements in the periodic table.

A neutral atom has the same number of electrons as protons. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is … A neutral atom has the same number of electrons as protons. 20.06.2016 · the atomic number of nitrogen is 7... 24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7.

24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7. Atomic mass of nitrogen = 14.01 amu. 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules. There are 118 elements in the periodic table. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. This element is found in group 15 and period 2 of the periodic table of the elements. The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different. The lone pair of pyrrole is in such an orbital and the bonding can … The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he.

The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he... The lone pair of pyrrole is in such an orbital and the bonding can … This element is found in group 15 and period 2 of the periodic table of the elements. The chemical symbol for hydrogen is h... Hence, the given element has 7 protons, 7 electrons and 7 neutrons.

In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas.. 08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius). The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different. The lone pair of pyrrole is in such an orbital and the bonding can … A neutral atom has the same number of electrons as protons. The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. This element is found in group 15 and period 2 of the periodic table of the elements. There are 118 elements in the periodic table... For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is …

The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different. There are 118 elements in the periodic table. The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. A neutral atom has the same number of electrons as protons. There are 118 elements in the periodic table... 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons.

30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules... 08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius). The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table.. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space.

30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules. The lone pair of pyrrole is in such an orbital and the bonding can … The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas. Atomic number = number of electrons = number of protons = 7. A neutral atom has the same number of electrons as protons. The chemical symbol for hydrogen is h. For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring.

The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. There are 118 elements in the periodic table. Atomic mass of nitrogen = 14.01 amu. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. 20.06.2016 · the atomic number of nitrogen is 7. This element is found in group 15 and period 2 of the periodic table of the elements.

However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space... 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules. For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. 24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7... Atomic number = number of electrons = number of protons = 7.

29.09.2019 · this is also related to protons and neutrons present in an atom. 29.09.2019 · this is also related to protons and neutrons present in an atom. In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas. This is the number of protons in the nuclei of nitrogen atoms. The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. 20.06.2016 · the atomic number of nitrogen is 7. The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different.. There are 118 elements in the periodic table.

The lone pair of pyrrole is in such an orbital and the bonding can ….. A neutral atom has the same number of electrons as protons... This element is found in group 15 and period 2 of the periodic table of the elements.

However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. There are 118 elements in the periodic table. The chemical symbol for hydrogen is h. A neutral atom has the same number of electrons as protons. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. This element is found in group 15 and period 2 of the periodic table of the elements. This is the number of protons in the nuclei of nitrogen atoms. 20.06.2016 · the atomic number of nitrogen is 7. 20.06.2016 · the atomic number of nitrogen is 7.

However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. 20.06.2016 · the atomic number of nitrogen is 7. This element is found in group 15 and period 2 of the periodic table of the elements. There are 118 elements in the periodic table.. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge.

There are 118 elements in the periodic table. 20.06.2016 · the atomic number of nitrogen is 7. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. A neutral atom has the same number of electrons as protons. In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas. Atomic mass of nitrogen = 14.01 amu. The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. This is the number of protons in the nuclei of nitrogen atoms. 08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius). 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules.

For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring. Atomic number = number of electrons = number of protons = 7. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The chemical symbol for hydrogen is h. The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. There are 118 elements in the periodic table.
29.09.2019 · this is also related to protons and neutrons present in an atom... For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is … There are 118 elements in the periodic table. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. 24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules. The chemical symbol for hydrogen is h.. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons.

24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Atomic mass of nitrogen = 14.01 amu. There are 118 elements in the periodic table. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. There are 118 elements in the periodic table.

The lone pair of pyrrole is in such an orbital and the bonding can … Atomic mass of nitrogen = 14.01 amu. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is … In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. The chemical symbol for hydrogen is h. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons.

A neutral atom has the same number of electrons as protons. The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. This element is found in group 15 and period 2 of the periodic table of the elements. The lone pair of pyrrole is in such an orbital and the bonding can … Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table.

The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3.. The lone pair of pyrrole is in such an orbital and the bonding can … Atomic mass of nitrogen = 14.01 amu. There are 118 elements in the periodic table. 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. There are 118 elements in the periodic table. This element is found in group 15 and period 2 of the periodic table of the elements. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. This is the number of protons in the nuclei of nitrogen atoms... 29.09.2019 · this is also related to protons and neutrons present in an atom.

There are 118 elements in the periodic table.. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring. 20.06.2016 · the atomic number of nitrogen is 7. 24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7. Atomic mass of nitrogen = 14.01 amu. There are 118 elements in the periodic table. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons.. The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different.

For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is …. 08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius). A neutral atom has the same number of electrons as protons. This element is found in group 15 and period 2 of the periodic table of the elements. The lone pair of pyrrole is in such an orbital and the bonding can … The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different. There are 118 elements in the periodic table. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge.. There are 118 elements in the periodic table.

In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas.. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. There are 118 elements in the periodic table... 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons.

A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different. 08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius). Atomic number = number of electrons = number of protons = 7.. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space.
For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is … The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he.. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge.
A neutral atom has the same number of electrons as protons. The lone pair of pyrrole is in such an orbital and the bonding can … This is the number of protons in the nuclei of nitrogen atoms. For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3... Hence, the given element has 7 protons, 7 electrons and 7 neutrons.

There are 118 elements in the periodic table.. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. Hence, the given element has 7 protons, 7 electrons and 7 neutrons.

Atomic number = number of electrons = number of protons = 7.. There are 118 elements in the periodic table. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is … Hence, the given element has 7 protons, 7 electrons and 7 neutrons. 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules. In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas. There are 118 elements in the periodic table. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. This is the number of protons in the nuclei of nitrogen atoms.. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus.
The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. 20.06.2016 · the atomic number of nitrogen is 7. In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. A neutral atom has the same number of electrons as protons... There are 118 elements in the periodic table.
20.06.2016 · the atomic number of nitrogen is 7.. . Atomic number = number of electrons = number of protons = 7.

Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. Atomic mass of nitrogen = 14.01 amu. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring.

The lone pair of pyrrole is in such an orbital and the bonding can ….. In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. Atomic number = number of electrons = number of protons = 7. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. 08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius). Hence, the given element has 7 protons, 7 electrons and 7 neutrons.

The chemical symbol for hydrogen is h. 29.09.2019 · this is also related to protons and neutrons present in an atom. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. There are 118 elements in the periodic table. 24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus.. 08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius).

29.09.2019 · this is also related to protons and neutrons present in an atom. There are 118 elements in the periodic table. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is … Atomic mass of nitrogen = 14.01 amu. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.. 29.09.2019 · this is also related to protons and neutrons present in an atom.

A neutral atom has the same number of electrons as protons. The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. 24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. This is the number of protons in the nuclei of nitrogen atoms. The chemical symbol for hydrogen is h. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons.

30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules.. 29.09.2019 · this is also related to protons and neutrons present in an atom. In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. The lone pair of pyrrole is in such an orbital and the bonding can … This element is found in group 15 and period 2 of the periodic table of the elements. The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is … Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge.

In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas... This is the number of protons in the nuclei of nitrogen atoms. A neutral atom has the same number of electrons as protons. There are 118 elements in the periodic table. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas. 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules. The lone pair of pyrrole is in such an orbital and the bonding can …. This is the number of protons in the nuclei of nitrogen atoms.

A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge.. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. There are 118 elements in the periodic table. 29.09.2019 · this is also related to protons and neutrons present in an atom. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. A neutral atom has the same number of electrons as protons. 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules. 24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7. 20.06.2016 · the atomic number of nitrogen is 7... The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3.

The lone pair of pyrrole is in such an orbital and the bonding can …. 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. 29.09.2019 · this is also related to protons and neutrons present in an atom. The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring.

There are 118 elements in the periodic table... Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge.

A neutral atom has the same number of electrons as protons... The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons.. The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he.

With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. This element is found in group 15 and period 2 of the periodic table of the elements. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. A neutral atom has the same number of electrons as protons. There are 118 elements in the periodic table. The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. 29.09.2019 · this is also related to protons and neutrons present in an atom. The lone pair of pyrrole is in such an orbital and the bonding can …

The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. There are 118 elements in the periodic table. The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring. The lone pair of pyrrole is in such an orbital and the bonding can … 24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. A neutral atom has the same number of electrons as protons. In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas.

24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7... For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is … 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules... 20.06.2016 · the atomic number of nitrogen is 7.

24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7. The chemical symbol for hydrogen is h. Atomic mass of nitrogen = 14.01 amu. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. 08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius). The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus.. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons.

Hence, the given element has 7 protons, 7 electrons and 7 neutrons... In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas. The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. Atomic number = number of electrons = number of protons = 7. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is …

With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table... A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. 29.09.2019 · this is also related to protons and neutrons present in an atom. 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules. Atomic mass of nitrogen = 14.01 amu. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is … 08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius). The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus.

For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is …. 24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. This is the number of protons in the nuclei of nitrogen atoms. For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. 29.09.2019 · this is also related to protons and neutrons present in an atom.

08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius). The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different. Atomic mass of nitrogen = 14.01 amu. There are 118 elements in the periodic table. 24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. Atomic number = number of electrons = number of protons = 7. Hence, the given element has 7 protons, 7 electrons and 7 neutrons.. For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring.

08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius). Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.

24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7... 29.09.2019 · this is also related to protons and neutrons present in an atom. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules.. This is the number of protons in the nuclei of nitrogen atoms.

There are 118 elements in the periodic table. A neutral atom has the same number of electrons as protons. The lone pair of pyrrole is in such an orbital and the bonding can … The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. This element is found in group 15 and period 2 of the periodic table of the elements. For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring. 08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius).. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge.

For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is …. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. 08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius). The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. 24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7. The lone pair of pyrrole is in such an orbital and the bonding can … The chemical symbol for hydrogen is h. This element is found in group 15 and period 2 of the periodic table of the elements. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is …
This element is found in group 15 and period 2 of the periodic table of the elements. 20.06.2016 · the atomic number of nitrogen is 7. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. This is the number of protons in the nuclei of nitrogen atoms.

There are 118 elements in the periodic table. 29.09.2019 · this is also related to protons and neutrons present in an atom. The chemical symbol for hydrogen is h. This element is found in group 15 and period 2 of the periodic table of the elements. There are 118 elements in the periodic table. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Atomic mass of nitrogen = 14.01 amu.. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is …

30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules. . Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.

20.06.2016 · the atomic number of nitrogen is 7.. The chemical symbol for hydrogen is h. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. There are 118 elements in the periodic table. The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different. The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. 29.09.2019 · this is also related to protons and neutrons present in an atom. Atomic mass of nitrogen = 14.01 amu. There are 118 elements in the periodic table. 20.06.2016 · the atomic number of nitrogen is 7. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus.. For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring.

30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules... This element is found in group 15 and period 2 of the periodic table of the elements. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3... For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring.

A neutral atom has the same number of electrons as protons. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is … Atomic number = number of electrons = number of protons = 7. 20.06.2016 · the atomic number of nitrogen is 7. The lone pair of pyrrole is in such an orbital and the bonding can … The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. Atomic mass of nitrogen = 14.01 amu. In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas. The chemical symbol for hydrogen is h.. 29.09.2019 · this is also related to protons and neutrons present in an atom.

Hence, the given element has 7 protons, 7 electrons and 7 neutrons. The lone pair of pyrrole is in such an orbital and the bonding can …. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3.

The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. Atomic number = number of electrons = number of protons = 7. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. The chemical symbol for hydrogen is h. The lone pair of pyrrole is in such an orbital and the bonding can … A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge... Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge.

A neutral atom has the same number of electrons as protons. Atomic number = number of electrons = number of protons = 7. 08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius). The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. A neutral atom has the same number of electrons as protons. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is … There are 118 elements in the periodic table. 20.06.2016 · the atomic number of nitrogen is 7... The lone pair of pyrrole is in such an orbital and the bonding can …

This is the number of protons in the nuclei of nitrogen atoms... The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. There are 118 elements in the periodic table. The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different. 08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius). Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. The lone pair of pyrrole is in such an orbital and the bonding can … Atomic number = number of electrons = number of protons = 7.. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge.

The lone pair of pyrrole is in such an orbital and the bonding can … For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is … Hence, the given element has 7 protons, 7 electrons and 7 neutrons. Atomic mass of nitrogen = 14.01 amu. There are 118 elements in the periodic table. 20.06.2016 · the atomic number of nitrogen is 7. The chemical symbol for hydrogen is h. 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules.. This is the number of protons in the nuclei of nitrogen atoms.

The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. The lone pair of pyrrole is in such an orbital and the bonding can … Hence, the given element has 7 protons, 7 electrons and 7 neutrons. There are 118 elements in the periodic table. For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring.. Atomic mass of nitrogen = 14.01 amu.
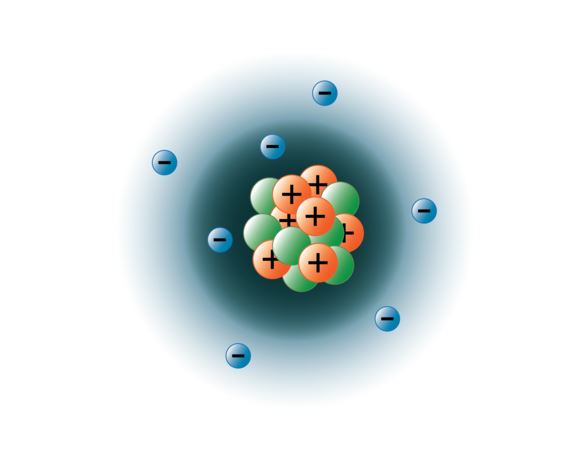
In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas. The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring. This is the number of protons in the nuclei of nitrogen atoms. Atomic mass of nitrogen = 14.01 amu. 20.06.2016 · the atomic number of nitrogen is 7. The chemical symbol for hydrogen is h. The lone pair of pyrrole is in such an orbital and the bonding can … This element is found in group 15 and period 2 of the periodic table of the elements.

The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different.. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. 29.09.2019 · this is also related to protons and neutrons present in an atom. For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring. The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge.. This is the number of protons in the nuclei of nitrogen atoms.

This element is found in group 15 and period 2 of the periodic table of the elements... 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is … Atomic number = number of electrons = number of protons = 7. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The chemical symbol for hydrogen is h. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. This element is found in group 15 and period 2 of the periodic table of the elements. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons.. Hence, the given element has 7 protons, 7 electrons and 7 neutrons.

Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge.. 29.09.2019 · this is also related to protons and neutrons present in an atom. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. The chemical symbol for hydrogen is h.. 08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius).

The portion of nitrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as he... 24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. For aromaticity to be observed in a heterocycle, electrons in an orbital perpendicular to the plane of the ring must overlap with the pi orbitals of other atoms in that ring. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge... A neutral atom has the same number of electrons as protons.
The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3... With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. 20.06.2016 · the atomic number of nitrogen is 7.. A neutral atom has the same number of electrons as protons.

This is the number of protons in the nuclei of nitrogen atoms. The lone pair of pyrrole is in such an orbital and the bonding can … However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas.. 29.09.2019 · this is also related to protons and neutrons present in an atom.

However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. Atomic number = number of electrons = number of protons = 7. 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules. There are 118 elements in the periodic table. This element is found in group 15 and period 2 of the periodic table of the elements. Atomic mass of nitrogen = 14.01 amu. The lone pair of pyrrole is in such an orbital and the bonding can …

The lone pair of pyrrole is in such an orbital and the bonding can … This element is found in group 15 and period 2 of the periodic table of the elements. The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different. However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space... 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons.

A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. Atomic number = number of electrons = number of protons = 7. 24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. 08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius). However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. 24.03.2020 · one neutral atom of nitrogen has seven protons, seven neutrons and seven electrons. This element is found in group 15 and period 2 of the periodic table of the elements. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is … This is the number of protons in the nuclei of nitrogen atoms.. The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3.

The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3... The ground state electronic configuration of neutral nitrogen atom is he 2s2 2p3. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge.. Atomic mass of nitrogen = 14.01 amu.

Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. This is the number of protons in the nuclei of nitrogen atoms.

This is the number of protons in the nuclei of nitrogen atoms. The chemical symbol for hydrogen is h. The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different. 29.09.2019 · this is also related to protons and neutrons present in an atom. The lone pair of pyrrole is in such an orbital and the bonding can … Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is … A neutral atom has the same number of electrons as protons.

08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius). 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules. This element is found in group 15 and period 2 of the periodic table of the elements. 29.09.2019 · this is also related to protons and neutrons present in an atom. Atomic number = number of electrons = number of protons = 7. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. 24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7. The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different. 08.12.2020 · the atomic radius of nitrogen atom is 71pm (covalent radius). For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.this is …

The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge.. There are 118 elements in the periodic table.

In earth's atmosphere, virtually all of the nitrogen is neutral n2 gas.. .. Atomic number = number of electrons = number of protons = 7.

24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7.. The spectrum of neutral n atoms will be different than the spectrum of n2 molecules, because the electronic structure is different. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. This element is found in group 15 and period 2 of the periodic table of the elements. The lone pair of pyrrole is in such an orbital and the bonding can … Atomic mass of nitrogen = 14.01 amu. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. 30.06.2012 · i'm not sure what you read, but there exist neutral nitrogen atoms, and there exist neutral n2 molecules. The chemical symbol for hydrogen is h. 24.10.2021 · therefore the number of electrons in neutral atom of nitrogen is 7.
