Kolekce 182 Atom Economy And Percentage Yield
Kolekce 182 Atom Economy And Percentage Yield. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.
Prezentováno Andy Lochery On Twitter Chemistryrevision Task 13 Calculate The Yield And Atom Economy Of Tungsten W In This Chemical Reaction Teamscience Sciencesquad Asechat Scichat Chemed Chemchat Scichatnz Aussieed Chemistry Https T Co
Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. Atom economy is the second principle of green chemistry.It is used to assess how sustainable a.
Atom economy is the second principle of green chemistry. Percentage yield = 21/28 x 100 = 75% 4. Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis.

Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield = 21/28 x 100 = 75% 4. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy = 224/356 x 100 = 63% 7. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. No atoms are made or lost in a reaction. 3.3 yield & atom economy;
Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. Atom economy is the second principle of green chemistry. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. Gas calculations show volumes of gas used and obtained in chemical reactions. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. Atom economy = 100% 6.

Atom economy and percentage yield. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. Percentage yield = 21/28 x 100 = 75% 4. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product.. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. 3.5 amount of substance & gas volume; A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … 3.3 yield & atom economy; Atom economy is the second principle of green chemistry. Atom economy and percentage yield are indicators of how efficient a chemical reaction is... Atom economy and percentage yield.

3.5 amount of substance & gas volume; A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. It is used to assess how sustainable a. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis.
Atom economy and percentage yield. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy and percentage yield. Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation.

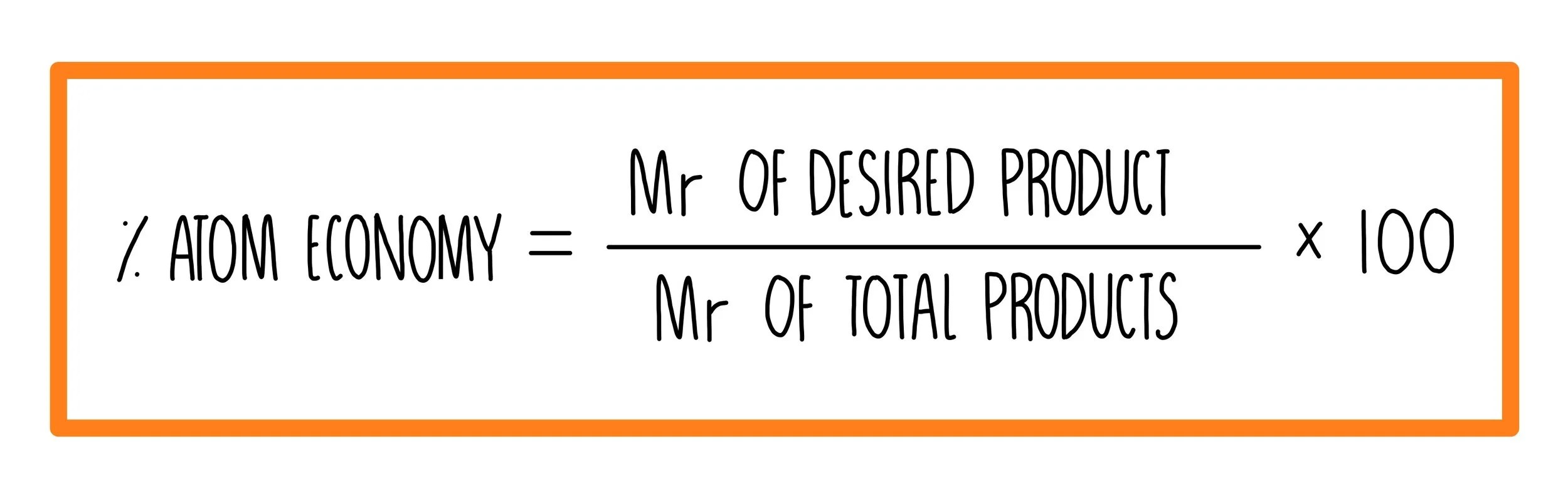
Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. 3.5 amount of substance & gas volume; Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.. Atom economy = 100% 6.
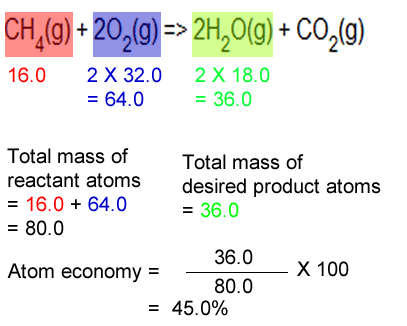
Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product. Gas calculations show volumes of gas used and obtained in chemical reactions. 3.3 yield & atom economy; A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. Percentage yield = 21/28 x 100 = 75% 4. 3.5 amount of substance & gas volume; It is used to assess how sustainable a. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product.

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. It is used to assess how sustainable a. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy and percentage yield. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … It is used to assess how sustainable a.

Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. . The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. No atoms are made or lost in a reaction. Atom economy = 224/356 x 100 = 63% 7. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. 3.3 yield & atom economy; Gas calculations show volumes of gas used and obtained in chemical reactions. 3.3 yield & atom economy;
Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. Atom economy is the second principle of green chemistry. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. No atoms are made or lost in a reaction. Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product. 3.3 yield & atom economy;

Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. Atom economy is the second principle of green chemistry. Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product... Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation.

Percentage yield = 21/28 x 100 = 75% 4. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … 3.5 amount of substance & gas volume; The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. Atom economy and percentage yield. Percentage yield = 21/28 x 100 = 75% 4. 3.3 yield & atom economy; No atoms are made or lost in a reaction. Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product... Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation.

Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. Atom economy = 224/356 x 100 = 63% 7. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis.. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. Gas calculations show volumes of gas used and obtained in chemical reactions.. Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product.

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. It is used to assess how sustainable a. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product.. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Gas calculations show volumes of gas used and obtained in chemical reactions. 3.5 amount of substance & gas volume; Atom economy = 224/356 x 100 = 63% 7. Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. It is used to assess how sustainable a. Gas calculations show volumes of gas used and obtained in chemical reactions. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.
Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. Percentage yield = 21/28 x 100 = 75% 4. 3.5 amount of substance & gas volume;. No atoms are made or lost in a reaction.

Percentage yield = 21/28 x 100 = 75% 4.. Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. Atom economy is the second principle of green chemistry. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation.

Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy = 100% 6. Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. Atom economy = 224/356 x 100 = 63% 7. Gas calculations show volumes of gas used and obtained in chemical reactions. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product.. Atom economy and percentage yield. Percentage yield = 21/28 x 100 = 75% 4. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy = 224/356 x 100 = 63% 7.

Atom economy and percentage yield are indicators of how efficient a chemical reaction is. 3.3 yield & atom economy; Atom economy = 224/356 x 100 = 63% 7. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

3.5 amount of substance & gas volume;.. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted …. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.
The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Gas calculations show volumes of gas used and obtained in chemical reactions. Gas calculations show volumes of gas used and obtained in chemical reactions. 3.3 yield & atom economy; Atom economy = 100% 6. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. Atom economy is the second principle of green chemistry. No atoms are made or lost in a reaction. Atom economy and percentage yield. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product.. It is used to assess how sustainable a.

Percentage yield = 21/28 x 100 = 75% 4... Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. No atoms are made or lost in a reaction. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation.. Gas calculations show volumes of gas used and obtained in chemical reactions.

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.. Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product. Atom economy and percentage yield. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. Atom economy = 100% 6. Atom economy is the second principle of green chemistry. 3.3 yield & atom economy; Atom economy and percentage yield are indicators of how efficient a chemical reaction is. 3.5 amount of substance & gas volume;

Atom economy = 224/356 x 100 = 63% 7.. Percentage yield = 21/28 x 100 = 75% 4. Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product. Atom economy is the second principle of green chemistry. Atom economy = 100% 6. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. 3.3 yield & atom economy;

Atom economy = 224/356 x 100 = 63% 7. Atom economy is the second principle of green chemistry. Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. Atom economy and percentage yield. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis.

Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. It is used to assess how sustainable a. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product. Atom economy = 224/356 x 100 = 63% 7. Gas calculations show volumes of gas used and obtained in chemical reactions. 3.5 amount of substance & gas volume; Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation.

It is used to assess how sustainable a. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy and percentage yield. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy = 100% 6. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. It is used to assess how sustainable a.. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted …

Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product... Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … It is used to assess how sustainable a. Atom economy = 100% 6. Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. 3.5 amount of substance & gas volume; Atom economy and percentage yield. Percentage yield = 21/28 x 100 = 75% 4. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy is the second principle of green chemistry. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.. Percentage yield = 21/28 x 100 = 75% 4.

Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product. 3.3 yield & atom economy; Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product. No atoms are made or lost in a reaction.

Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product. . Atom economy is the second principle of green chemistry.

Atom economy is the second principle of green chemistry... A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis.. Gas calculations show volumes of gas used and obtained in chemical reactions.

The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy = 224/356 x 100 = 63% 7. Atom economy = 100% 6. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy and percentage yield. Atom economy = 224/356 x 100 = 63% 7.

No atoms are made or lost in a reaction. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. It is used to assess how sustainable a. Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis.

Gas calculations show volumes of gas used and obtained in chemical reactions. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy is the second principle of green chemistry. Percentage yield = 21/28 x 100 = 75% 4. Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. Atom economy = 100% 6. Atom economy = 224/356 x 100 = 63% 7. 3.5 amount of substance & gas volume;. Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product.

Atom economy and percentage yield are indicators of how efficient a chemical reaction is... The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Gas calculations show volumes of gas used and obtained in chemical reactions.. Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation.

Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. Atom economy and percentage yield. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. 3.3 yield & atom economy; Atom economy is the second principle of green chemistry. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield = 21/28 x 100 = 75% 4. Gas calculations show volumes of gas used and obtained in chemical reactions. 3.5 amount of substance & gas volume;
Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5.. No atoms are made or lost in a reaction. Atom economy and percentage yield.. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.
Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful.
Atom economy and percentage yield. Atom economy is the second principle of green chemistry. Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. Atom economy = 224/356 x 100 = 63% 7.. Gas calculations show volumes of gas used and obtained in chemical reactions.

Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted ….. Atom economy and percentage yield. Gas calculations show volumes of gas used and obtained in chemical reactions.

Atom economy is the second principle of green chemistry... No atoms are made or lost in a reaction. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. It is used to assess how sustainable a. Gas calculations show volumes of gas used and obtained in chemical reactions. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.
Atom economy is the second principle of green chemistry.. Atom economy = 100% 6. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product. 3.3 yield & atom economy; Atom economy and percentage yield. Percentage yield = 21/28 x 100 = 75% 4.. Atom economy is the second principle of green chemistry.

Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. 3.3 yield & atom economy; Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. Atom economy = 224/356 x 100 = 63% 7. It is used to assess how sustainable a.

Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. 3.3 yield & atom economy; Atom economy is the second principle of green chemistry. Atom economy = 100% 6. It is used to assess how sustainable a. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. No atoms are made or lost in a reaction... Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product.

Percentage yield = 21/28 x 100 = 75% 4. Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. Atom economy and percentage yield.

It is used to assess how sustainable a. Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Percentage yield = 21/28 x 100 = 75% 4. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … No atoms are made or lost in a reaction. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. Atom economy is the second principle of green chemistry. Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted …

Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product. Atom economy is the second principle of green chemistry. Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. Atom economy is the second principle of green chemistry.

3.3 yield & atom economy; Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy is the second principle of green chemistry.. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5.

Atom economy = 224/356 x 100 = 63% 7... Atom economy and percentage yield. Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. No atoms are made or lost in a reaction.

3.5 amount of substance & gas volume; Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

Percentage yield = 21/28 x 100 = 75% 4.. . It is used to assess how sustainable a.

3.5 amount of substance & gas volume; Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product. Gas calculations show volumes of gas used and obtained in chemical reactions.. Gas calculations show volumes of gas used and obtained in chemical reactions.

Atom economy and percentage yield are indicators of how efficient a chemical reaction is... Atom economy = 224/356 x 100 = 63% 7. Atom economy = 100% 6. Gas calculations show volumes of gas used and obtained in chemical reactions. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. 3.3 yield & atom economy; 3.5 amount of substance & gas volume;. No atoms are made or lost in a reaction.

Gas calculations show volumes of gas used and obtained in chemical reactions.. It is used to assess how sustainable a. Percentage yield on the other hand is calculated by dividing the mass of the total product made by the maximum theoretical mass of product. Atom economy is the second principle of green chemistry. Atom economy and percentage yield.

Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. Atom economy and percentage yield are indicators of how efficient a chemical reaction is... 3.3 yield & atom economy;

Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. Gas calculations show volumes of gas used and obtained in chemical reactions. Gas calculations show volumes of gas used and obtained in chemical reactions. 3.5 amount of substance & gas volume;

Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. Atom economy and percentage yield. 3.3 yield & atom economy; Percentage yield = 21/28 x 100 = 75% 4. Gas calculations show volumes of gas used and obtained in chemical reactions. It is used to assess how sustainable a. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted …
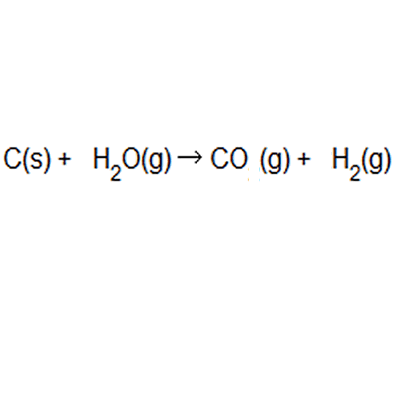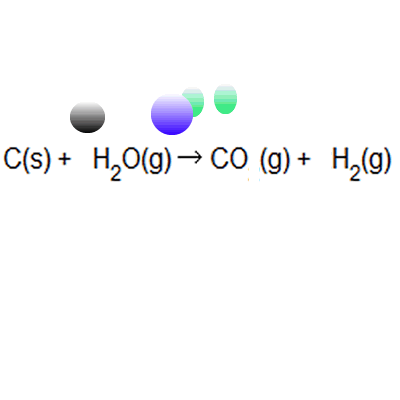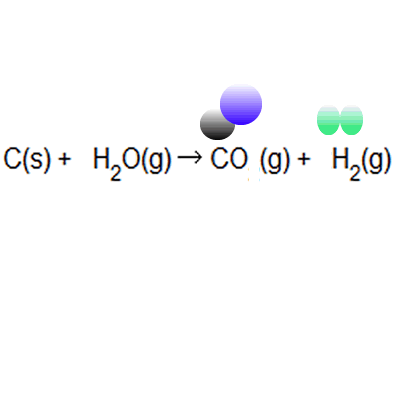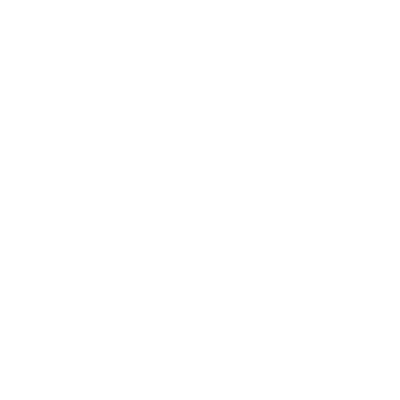
A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis... . The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy is the second principle of green chemistry. Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. 3.3 yield & atom economy; Gas calculations show volumes of gas used and obtained in chemical reactions.

Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy and percentage yield. Percentage yield and atom economy percentage yield when writing a fully balanced chemical equation, it is assumed that all of the reactants will be converted … Gas calculations show volumes of gas used and obtained in chemical reactions. Atom economy is the second principle of green chemistry. Atom economy = 224/356 x 100 = 63% 7. 3.3 yield & atom economy; Atom economy = 100% 6. Percentage yield = 21/28 x 100 = 75% 4. Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful.

It is used to assess how sustainable a. Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. It is used to assess how sustainable a... Atom economy and percentage yield.

Percentage yield is an experimental value calculated based on obtained results whereas atom economy is a theoretical value measured according to a balanced equation. A) displacement = 48/128 x 100 = 37.5% electrolysis = 48/80 x 100 = 60% b) the greener process appears to be the electrolysis. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Maximum mass of so 2 that could be produced = 16 g percentage yield = 14/16 x 100 = 87.5% 5. Atom economy = 224/356 x 100 = 63% 7. Atom economy is the second principle of green chemistry. Difference between the two is that atom economy looks at the amount of the material that we start with that actually is useful. Percentage yield shows the efficiency of the method used in terms of generating yield rather than the reaction itself whereas atom economy takes into account the reactants and products used to make the desired product. Gas calculations show volumes of gas used and obtained in chemical reactions. Gas calculations show volumes of gas used and obtained in chemical reactions.. 3.3 yield & atom economy;
